42 when were food labels required
Changes to the Nutrition Facts Label | FDA - U.S. Food and Drug ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ... Pure Food and Drug Act - Wikipedia Under the law, drug labels, for example, had to list any of 10 ingredients that were deemed "addictive" and/or "dangerous" on the product label if they were present, and could not list them if they were not present. Alcohol, morphine, opium, and cannabis were all included on the list of these "addictive" and/or "dangerous" drugs. The law also established a federal cadre of food …
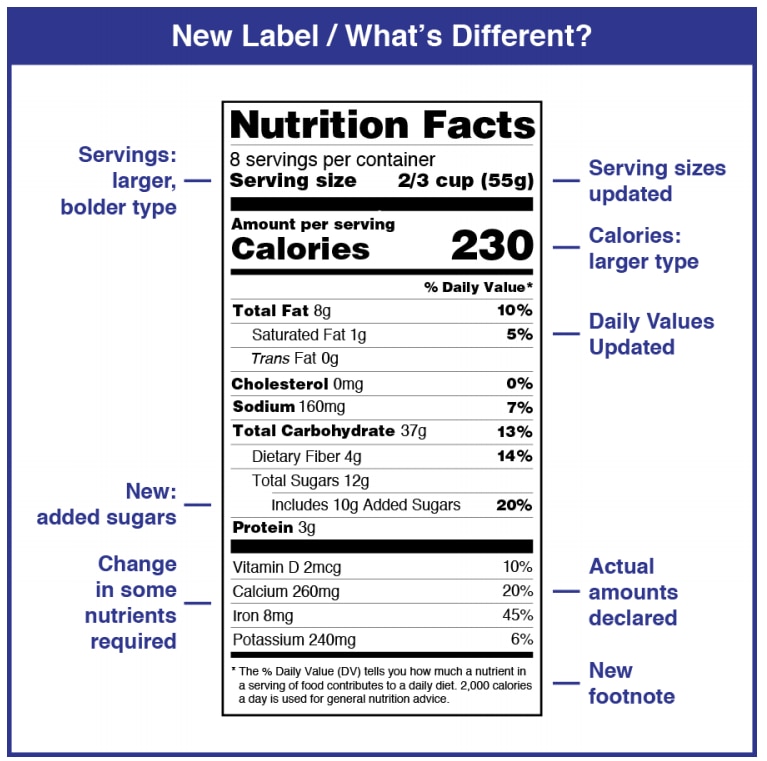
Added Sugars on the New Nutrition Facts Label | FDA - U.S. Food … 25/02/2022 · Labels on packages and containers of single-ingredient sugars and syrups such as table sugar, maple syrup, or honey will list the percent Daily Value for added sugars within the Nutrition Facts ...
When were food labels required
Food and Drug Administration - Wikipedia The United States Food and Drug Administration ... The nine new graphic warning labels were announced by the FDA in June 2011 and were scheduled to be required to appear on packaging by September 2012. The implementation date is uncertain, due to ongoing proceedings in the case of R.J. Reynolds Tobacco Co. v. U.S. Food and Drug Administration. R.J. Reynolds, … Important safety label changes to cholesterol-lowering statin drugs The U.S. Food and Drug Administration (FDA) has approved important safety label changes for the class of cholesterol-lowering drugs known as statins. Packaging and labeling - Wikipedia In 2019 the global food packaging market size was estimated at USD 303.26 billion, exhibiting a CAGR of 5.2% over the forecast period. Growing demand for packaged food by consumers owing to quickening pace of life and changing eating habits is expected to have a major impact on the market. The purposes of packaging and package labels
When were food labels required. Genetically modified food controversies - Wikipedia Public perception. Consumer concerns about food quality first became prominent long before the advent of GM foods in the 1990s. Upton Sinclair's novel The Jungle led to the 1906 Pure Food and Drug Act, the first major US legislation on the subject. This began an enduring concern over the purity and later "naturalness" of food that evolved from a single focus on sanitation to include … List of ingredients and allergens on food labels - Canadian Food ... Health Canada and the CFIA encourage food manufacturers and importers to use the title "May contain:" or "May contain" to introduce the cross-contamination statement on food labels. If a title is used, it must appear in bold when the statement appears on the same line as the ingredient list or the "food allergen source, gluten and added sulphites" statement [B.01.010.4(1)(d), FDR ]. National Bioengineered Food Disclosure Standard - Federal Register 21/12/2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. Consumer Reports Magazine 2022 Sign In. We don’t recognize that sign in. Your username maybe be your email address. Passwords are 6-20 characters with at least one number and letter.
Pet Food Labels - General | FDA Kilocalories are the same as the "Calories" consumers are used to seeing on food labels. A "kilogram" is a unit of metric measurement equal to 2.2 pounds. Manufacturers are also required to ... Home | Food Safety and Inspection Service The Food Safety and Inspection Service is responsible for ensuring that meat, poultry, Siluriformes, and eggs are safe and are properly labeled and packaged. Learn more about our inspection services and process. Packaging and labeling - Wikipedia In 2019 the global food packaging market size was estimated at USD 303.26 billion, exhibiting a CAGR of 5.2% over the forecast period. Growing demand for packaged food by consumers owing to quickening pace of life and changing eating habits is expected to have a major impact on the market. The purposes of packaging and package labels Important safety label changes to cholesterol-lowering statin drugs The U.S. Food and Drug Administration (FDA) has approved important safety label changes for the class of cholesterol-lowering drugs known as statins.
Food and Drug Administration - Wikipedia The United States Food and Drug Administration ... The nine new graphic warning labels were announced by the FDA in June 2011 and were scheduled to be required to appear on packaging by September 2012. The implementation date is uncertain, due to ongoing proceedings in the case of R.J. Reynolds Tobacco Co. v. U.S. Food and Drug Administration. R.J. Reynolds, …



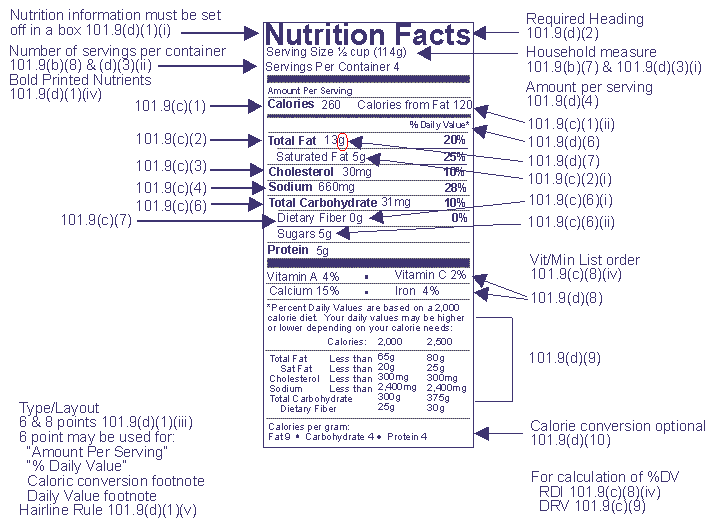







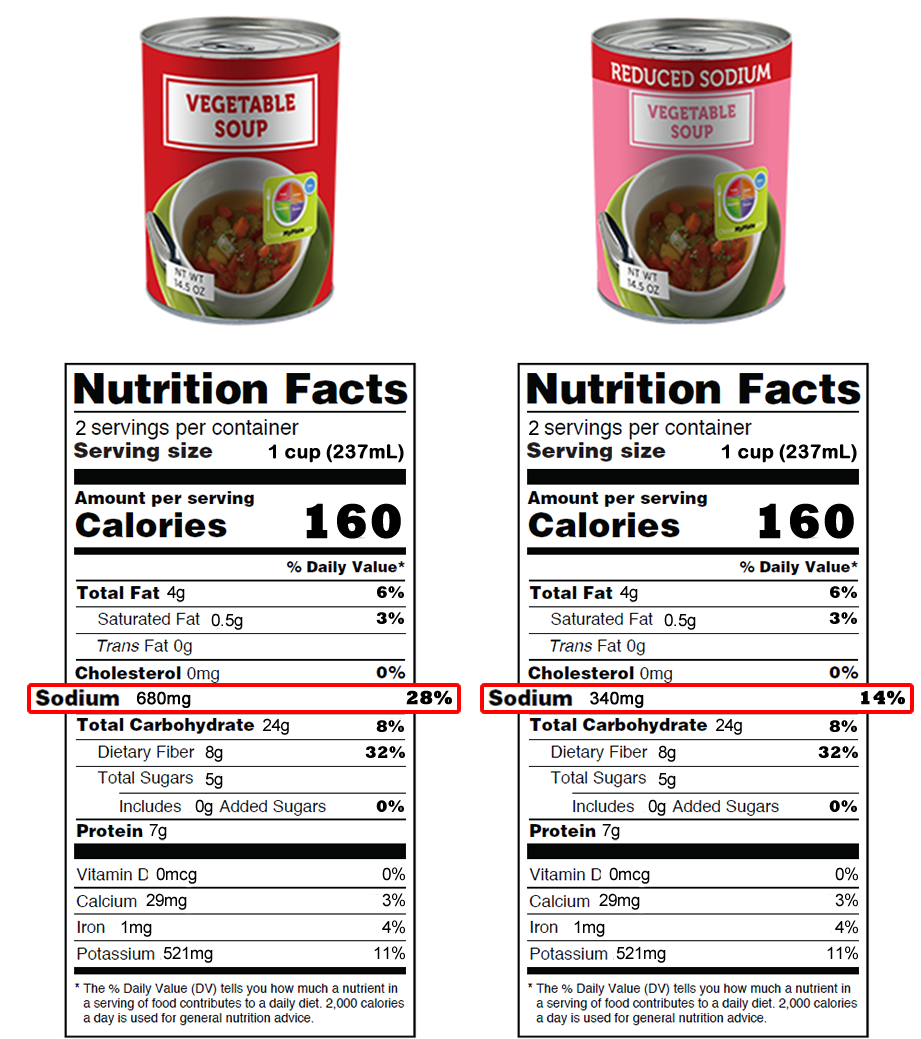





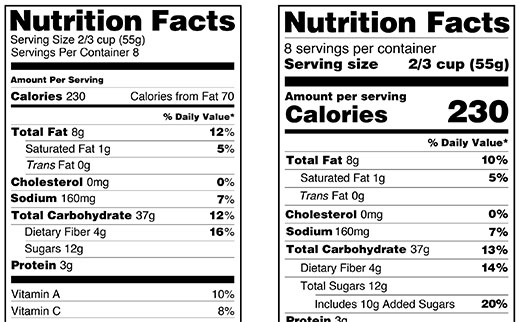



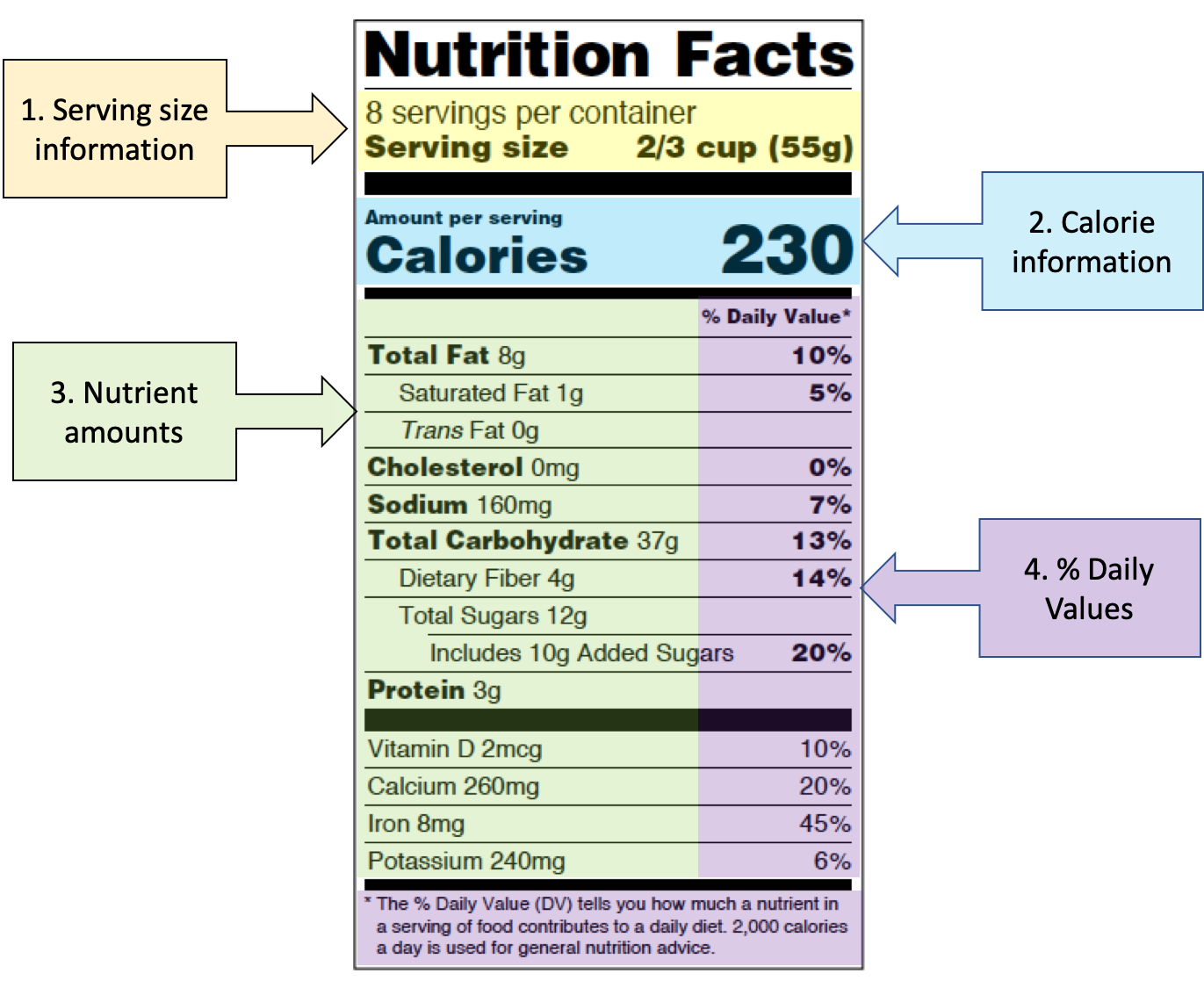










Post a Comment for "42 when were food labels required"